Williams Group research published in the Journal of the American Chemical Society
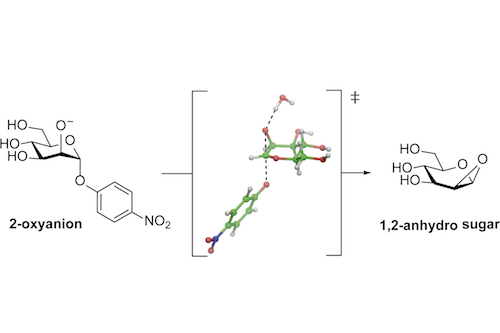
Glycosides are most commonly hydrolyzed through acid catalyzed processes. However, for over 100 years it has been recognized that certain naturally-occurring glycosides such as salicin (an anti-inflammatory agent from willow bark), undergo rapid cleavage in basic conditions. The mechanism involves a ‘back-biting’ neighbouring group participation in which an oxyanion of the 2-hydroxy group performs a nucleophilic attack, substituting the glycosidic linkage, to make a sugar epoxide (a 1,2-anhydro sugar), which is subsequently hydrolyzed.
Gaetano Speciale and Spencer Williams from the School of Chemistry, in collaboration with researchers at Simon Fraser University, have utilized kinetic isotope effect (KIE) analysis to probe the nature of the transition state of the alkaline solvolysis of 4-nitrophenyl alpha-mannoside (Speciale, G., Farren-Dai, M., Shidmoossavee, F., Williams, S.J., Bennet, A.J., J. Am. Chem. Soc., 2016, 138, 14012−14019). KIEs require accurate measurement of the effect upon rate of the reaction when extra neutrons are added to various atoms within the substrate to form isotopic isomers. The measured KIEs were modelled computationally, providing a picture of the shape and charge distribution of the transition state. The kinetic signatures have the potential to provide insight into the mechanism of enzymes that potentially cleave glycosides through an equivalent process.