Mannanases that take the road less travelled
Mannans are a diverse group of polysaccharides present within the plant cell wall and endosperm. Their degradation is catalyzed by β-1,4-mannanases and assorted auxiliary enzymes, which act in concert to liberate, ultimately, D-mannose. β-Mannanases are of increasing industrial significance in the food, detergent, and biofuels industries, which process β-mannan-rich plant material, and, in the oil and gas industries, which deploy β-1,4-mannan materials to modulate fluid rheology in fracking processes.
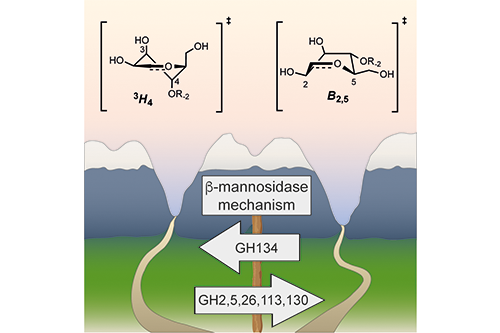
Marija Petricevic from the Williams Research Group, as part of a multinational team of researchers from the UK and Spain, has reported on a novel mechanism for a newly described family of β-mannanases, as well as a novel tertiary structure. A representative of this new family was shown to adopt a fold closely related to that of hen egg white lysozyme, which is historically significant as the first enzyme to have its three-dimensional structure determined. Biochemical, X-ray structural, and quantum mechanical methods were used to show that the enzyme performs catalysis with an inversion of anomeric configuration, and a so-called 'Southern hemisphere' itinerary involving the sugar ring in a ring-flipped conformation.