Exploring active site coordination geometry through enzymes and small molecule models
In a long running collaboration with Prof. David Goldberg (Johns Hopkins University), the Jameson lab, including PhD candidates Josh Prendergast and Zahra Shirani-Sarmazeh, have published a new study in the Journal of the American Chemical Society.
Cysteine dioxygenase oxidises cysteine to cysteine-sulfinate by addition of molecular oxygen at a ferrous iron held in place by three histidine residues from the protein. Substrate cysteine coordinates to the iron via the thiol and amine groups to produce a pseudo-octahedral iron complex with one coordination site free to bind the other substrate dioxygen.
In previous work (Tchesnokov et al. Chem. Commun. 2016), the Jameson Lab have shown through Mössbauer spectroscopy that the cysteine-bound enzyme exists as two species. Small molecule models designed by the Goldberg group have shown that the protein spectra can be explained as a mixture of 5- and 6-coordinate iron. Thus, in the protein the dioxygen binding site is partially occupied by a water molecule in stark contrast to other enzymes of this class.
The model complexes were shown to undergo similar chemistry to the enzyme at low temperatures, converting the non-native substrate 2-aminobenzenthiol to the corresponding sulfinate. In contrast the enzyme was unable to do this, emphasising the importance of correct substrate orientation to reactivity.
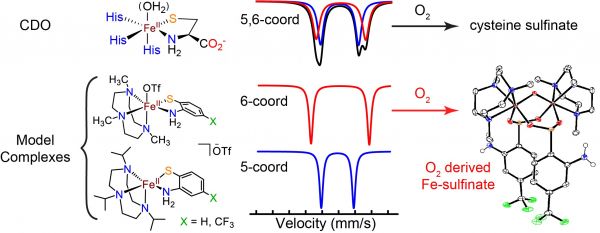
Gordon, J.B., McGale, J.P., Prendergast, J.R., Shirani-Sarmazeh, Z., Siegler, M.A., Jameson, G.N.L., Goldberg, D.P, J. Am. Chem. Soc., 2018, 140 (44), pp 14807–14822.
Read more here.