Progress toward extending human protein pumps
Protein pumps play a central role in metabolism of the nutrient metal copper by pumping copper(I) ions across cell membranes against the thermodynamic gradient using energy derived from ATP hydrolysis. Malfunction of the human pumps ATP7A and ATP7B are directly responsible for, respectively, Menkes and Wilson diseases and for a spectrum of neuro-pathologies. Membrane proteins are notoriously difficult to isolate intact as they feature both hydrophilic and hydrophobic domains. The laboratory of Zhiguang Xiao and Anthony Wedd (University of Melbourne) has isolated a bacterial analogue of the human pumps and characterised it in molecular detail (C J. K. Wijekoon, S. R. Udagedara, R. L. Knorr, R. Dimova, A. G. Wedd and Z. Xiao,J. Amer. Chem. Soc. 2017, 139, 4266–4269).
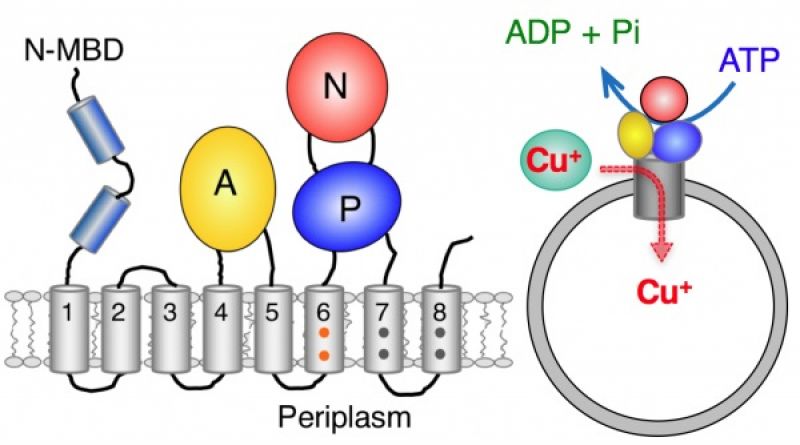
The pump was embedded in a closed membrane system (see cartoon) via a collaboration with Roland Knorr and Rumiana Dimova (Max Planck Institute of Colloids and Interfaces, Potsdam, Germany) and shown to transfer one copper(I) ion per ATP molecule hydrolysed. This is the first quantitative correlation of ATPase activity and copper translocation. The stoichiometry contrasts with the two Ca2+ ions pumped in the calcium ATPase pumps. Extension to the human copper pumps is now possible.